Molecular Thermometers
Our lab has been experimentating with "molecular thermometers," where we are making use of fluorophore blinking to measure temperature at the molecular level.
What are fluorophores and why do they blink?
Fluorophores are molecules which can absorb photons with a certain wavelength, and then emit photons with a different (longer) wavelength. Most fluorophores have states in which they are non-fluorescent (eg. triplet states), and can alternate between fluorescent and non-fluorescent states, causing blinking. In particular, the enhanced green fluorescent protein (EGFP) is fluorescent when excited at 488nm light only when its Tyrosine-66 hydroxyl group is not protonated. Reversible protonation thus causes "blinking" as the protein alternates between states (with relaxation times on the order of 100μs).
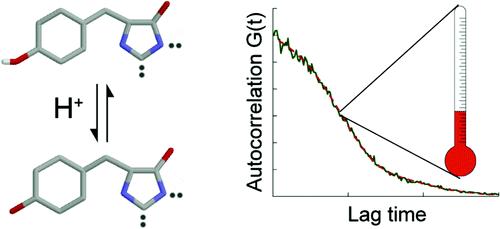
Below left: Tyrosine residues of EGFP alternating between its fluorescent and non-fluorescent states.
Below right: An autocorrelation function obtained by fluorescence correlation spectroscopy for EGFP, showing a small diffusion contribution and a large blinking contribution (from which temperature can be inferred). (Adapted from Wong et al...)
How can fluorophore blinking be used to measure temperature?
The rates at which molecules switch from their different states (eg. fluorescent to non-fluorescent) are affected by temperature. Therefore the relaxation time associated with an equilibrium between a fluorescent and a non-fluorescent species (blinking) is temperature dependent. There are other factors that affect molecular relaxation times such as pH, buffer components, and the specific reaction, but these can be carefully controlled in order ensure that there is a one-to-one relationship between blinking relaxtion time and temperature.
How can we detect fluorophore blinking?
Blinking (with relaxation times in the 1μs - 1ms range) results in fluctuation in the fluorescence signal emitted by the fluorophore, and can be detected by FCS, where blinking is evidenced by a short-time correlation appearing in the autocorrelation function (see above).
What are potential applications of this method?
This method is interesting compared to other fluorescence methods for measuring temperature because it provides absolute temperatures (~1°C resolution) regardless of excitation intensity, fluorophore concentration or fluorescence background in a spatially resolved manner (~1 μm resolution). It can therefore be used to measure temperature gradients in microfluids and biological systems. In particular, it has the potential to allow measuring a single cell's temperature.
References
Publications
- Wong, F. H., Fradin, C. (2011). Simultaneous pH and temperature measurements using pyranine as a molecular probe. Journal of Fluorescence, 21(1), 299-312. doi:10.1007/s10895-010-0717-y [Read]
- Wong, F., Banks, D. S., Abu-Arish, A., Fradin, C. (2007). A molecular thermometer based on fluorescent protein blinking. Journal of the American Chemical Society, 129(34), 10302-10303. doi:10.1021/ja0715905 [Read]
